Rifaximin
Reduces Encephalopathy Recurrence, Improves Quality of Life
in People with Liver Cirrhosis
 |
 |
 |
 |
 |
 |
 |
| SUMMARY:
The broad-spectrum antibiotic rifaximin
(Xifaxan) improves quality of life for people
with liver
cirrhosis who experience recurrent episodes of
hepatic encephalopathy, or brain disease, according
to research presented at the 45th Annual Meeting of
the European Association for the Study of the Liver
(EASL 2010) last month
in Vienna. Another analysis from the same study indicated
that rifaximin works by lowering the level of ammonia
in the blood. |
|
 |
 |
 |
 |
 |
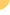 |
 |
By
Liz Highleyman
Hepatic encephalopathy is a form of neurocognitive impairment
caused by build-up of toxins such as ammonia -- thought to be
produced by bacteria in the gut -- in people with livers so
damaged that they cannot perform their normal filtering function
(known as decompensated cirrhosis). Over years or decades, chronic
hepatitis B and C can
progress to decompensated liver disease.
 Rifaximin
is a minimally absorbed broad-spectrum oral antibiotic that
concentrates in the gastrointestinal tract. It has been used
successfully to treat acute hepatic encephalopathy, and researchers
recently
reported that it maintained hepatic encephalopathy remission
better than placebo. In March, the U.S. Food and Drug Administration
(FDA) announced the approval
of rifaximin for prevention of hepatic encephalopathy recurrence.
Rifaximin
is a minimally absorbed broad-spectrum oral antibiotic that
concentrates in the gastrointestinal tract. It has been used
successfully to treat acute hepatic encephalopathy, and researchers
recently
reported that it maintained hepatic encephalopathy remission
better than placebo. In March, the U.S. Food and Drug Administration
(FDA) announced the approval
of rifaximin for prevention of hepatic encephalopathy recurrence.
Quality of Life
In the first analysis presented at EASL, Arun Sanyal and colleagues,
who conducted the aforementioned remission study, asked participants
to complete the Chronic Liver Disease Questionnaire (CLDQ),
a validated survey designed to assess health-related quality
of life in people with chronic liver disease.
The Phase 3 RFHE3001 trial included 299 patients in the U.S.,
Canada, and Russia with liver cirrhosis (MELD score <
25) who had experienced at least 2 episodes of recurrent hepatic
encephalopathy (Conn score > 2) during the past 6
months, but were currently in remission.
Participants were randomly assigned to receive either 550 mg
twice-daily rifaximin or placebo for 6 months. More than 90%
also received lactulose, a laxative commonly used to reduce
ammonia in the gut.
The present analysis included a subset of 219 patients (the
Americans and Canadians). About 70% were men, 80% were white,
and the average age was about 57 years. The mean MELD score
(a measure of liver disease severity) was 13.8 and 40% had experienced
more than 2 hepatic encephalopathy episodes during the past
6 months.
The CLDQ was administered at baseline, every 2 weeks until week
8, then every 4 weeks through the end of treatment. The survey
included 29 items in 6 domains: abdominal symptoms, fatigue,
systemic symptoms, activity, emotional function, and worry.
The overall score and the separate domain scores were ranked
on a scale of 1-7, with higher scores indicating better quality
of life.
Results
 |
Mean
time-weighted average scores for the overall CLDQ were significantly
higher in the rifaximin group compared with the placebo
group (3.7 vs 2.9; P = 0.009). |
 |
Participants
in the rifaximin arm also had significantly better average
scores on the separate domains: |
| |
 |
Fatigue
(higher = less fatigue): 3.2 with rifaximin vs 2.5
with placebo (P = 0.008); |
 |
Abdominal
symptoms: 4.1 vs 3.3, respectively (P = 0.009); |
 |
Systemic
symptoms: 3.9 vs 3.2, respectively (P = 0.016); |
 |
Activity:
3.7 vs 2.8, respectively (P = 0.002); |
 |
Emotional
function: 3.9 vs 3.0, respectively (P = 0.0065); |
 |
Worry:
3.5 vs 2.8, respectively (P = 0.0436). |
|
 |
Participants
who experienced breakthough hepatic encephalopathy (recurrence
despite treatment) had significantly worse quality of life
scores than those who stayed in remission. |
 |
Rifaximin
was generally well-tolerated, with a safety profile comparable
to that of placebo. |
Based
on these findings, the researchers concluded, "Rifaximin
550 mg twice-daily significantly improved quality of life overall
and across individual domains over a 6 month treatment period
in subjects with hepatic cirrhosis and recurrent, overt hepatic
encephalopathy."
Ammonia Levels
In the second reported analysis, Sanyal's team evaluated the
effect of rifaximin on venous (blood in veins) ammonia concentration
in the same study, and looked at the association between ammonia
concentration and breakthrough hepatic encephalopathy. Breakthrough
was defined as a Conn score rising to > 2 or Conn
score and asterixis (wrist tremor) grade each increasing by
1 point. Venous ammonia was measured at baseline and at days
24, 84, and 168 during treatment.
Results
 |
Out
of the 299 enrolled participants, 104 (34.8%) experienced
hepatic encephalopathy breakthrough and 194 (64.8%) maintained
remission. |
 |
Patients
receiving rifaximin experienced a significantly larger decrease
in venous ammonia concentration compared with placebo recipients
(mean change of -5.7 vs -1.2 mcg/dL, respectively; P = 0.039). |
 |
Participants
with higher time-weighted average ammonia concentrations
were significantly more likely to develop recurrent hepatic
encephalopathy (P = 0.007). |
"Rifaximin
therapy protected against breakthrough overt hepatic encephalopathy
and significantly decreased ammonia concentrations versus placebo,"
the investigators concluded. "The time-weighted average
of venous ammonia concentrations independently predicted breakthrough
hepatic encephalopathy in this 6 month study, underscoring the
reliability and clinical relevance of Conn score-measured breakthrough
hepatic encephalopathy episodes."
Investigator affiliations: Virginia Commonwealth University,
Richmond, VA; University of California, San Francisco, CA; University
of California at San Francisco, Fresno MEP, CA; Case Western
Reserve University, Cleveland, OH; Cedars-Sinai Medical Center,
Los Angeles, CA; Weill Medical College of Cornell University,
New York, NY; California Pacific Medical Center, San Francisco,
CA; Columbia University Medical Center, New York, NY; Delta
Research Partners, LLC, Monroe, LA; Gastroenterology Associates
of Central Georgia, Macon, GA; Salix Pharmaceuticals, Inc.,
Morrisville, NC.
5/28/10
References
Sanyal,
N Bass, K Mullen, and others. Rifaximin treatment improved quality
of life in patients with hepatic encephalopathy: results of
a large, randomized, placebo-controlled trial. 45th Annual Meeting
of the European Association for the Study of the Liver (EASL
2010). Vienna, Austria. April 14-18, 2010. (Abstract
15).
A
Sanyal, N Bass, F Poordad, and others. Rifaximin decreases venous
ammonia concentrations and time-weighted average ammonia concentrations
correlate with overt hepatic encephalopathy (HE) as assessed
by Conn score in a 6 month study. 45th Annual Meeting of the
European Association for the Study of the Liver (EASL 2010).
Vienna, Austria. April 14-18, 2010. (Abstract
195).