Genentech
Informs Customers of Important Information
about Triad Group's Alcohol Prep Pads
 |
Consumers
Alerted to Discontinue Use of Alcohol Prep Pads Packaged with
Boniva Injection, Fuzeon, Nutropin A.Q. Pen, Pegasys, TNKase
Medicines |
South
San Francisco, Calif. -- January 13, 2011 -- Genentech, Inc.,
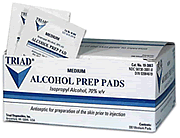
a member of the Roche Group, has become aware of the
market recall of Triad Group's alcohol prep pads, alcohol
swabs, and alcohol swabsticks manufactured by Triad in the
United States and marketed under various brand names. The
Triad Group alcohol prep pads are co-packaged and distributed
with Genentech medicines Boniva Injection, Fuzeon, Nutropin
A.Q. Pen, Pegasys, and TNKaseto customers in the United States.
According to the Food and Drug Administration's (FDA) Medwatch
communication, the recall was initiated due to concerns about
potential contamination of the Triad Group's products with
the bacteria, Bacillus cereus. This recall involves
those products marked as sterile as well as non-sterile. Use
of contaminated alcohol prep pads, alcohol swabs, and alcohol
swabsticks could lead to life-threatening infections, especially
in at-risk populations, including immune suppressed and surgical
patients.
It is important to note, that Genentech medicines are not
contaminated and may continue to be used in accordance with
the package insert. Patients and healthcare providers should
not use the alcohol prep pads packaged with these medicines
and should instead use an alternate alcohol prep pad that
is not involved with the Triad Group recall, or alternatively
use a sterile gauze pad in conjunction with isopropyl alcohol
for disinfecting the injection site prior to administration.
Genentech
is in discussion with the FDA and is currently assessing alternatives
to address the situation. The company plans to issue a Dear
Healthcare Provider letter to potential prescribers and pharmacists
to make them aware of the Triad product recall and the need
to discontinue use of the alcohol prep pads packaged with
Boniva Injection, Fuzeon, Nutropin A.Q. Pen, Pegasys, and
TNKase.
Patients should consult their healthcare provider for further
information. Healthcare providers with questions may contact
the Patient Resource Center at 1-877-436-3683 between the
hours of 6 a.m. and 5 p.m. Pacific Time.
 |
For
the Boniva indication, full prescribing information,
and important safety information, please visit www.boniva.com. |
 |
For
the Fuzeon indication, full prescribing information,
and important safety information, please visit www.fuzeon.com. |
 |
For
the Nutropin A.Q. Pen indication, full prescribing information,
and important safety information, please visit www.nutropin.com. |
 |
For
the Pegasys indication, full prescribing information,
and important safety information including Boxed Warning
and Medication Guide, please visit www.pegasys.com. |
 |
For
the TNKase indication, full prescribing information,
and important safety information, please visit www.tnkase.com. |
 Founded
more than 30 years ago, Genentech is a leading biotechnology
company that discovers, develops, manufactures and commercializes
medicines to treat patients with serious or life-threatening
medical conditions. The company, a member of the Roche Group,
has headquarters in South San Francisco, California. For additional
information about the company, please visit http://www.gene.com. Founded
more than 30 years ago, Genentech is a leading biotechnology
company that discovers, develops, manufactures and commercializes
medicines to treat patients with serious or life-threatening
medical conditions. The company, a member of the Roche Group,
has headquarters in South San Francisco, California. For additional
information about the company, please visit http://www.gene.com.
1/18/11
Sources
Genentech.
Genentech Informs Customers of Important Information about
Triad Group's Alcohol Prep Pads. Media advisory. January 13,
2010.
FDA.
Triad Group Issues a Voluntary Nationwide Recall of All Lots
of Alcohol Prep Pads, Alcohol Swabs, and Alcohol Swabsticks
Due to Potential Microbial Contamination. Press release. January
5, 2011.
|
