FDA
Committee Unanimously Votes to Approve Tesamorelin (Egrifta) for
Lipodystrophy
 |
 |
 |
 |
 |
 |
 |
| SUMMARY:
The U.S. Food and Drug Administration (FDA) Endocrinologic
and Metabolic Drugs Advisory Committee recommended by
a 16-0 vote last Thursday that the agency should approve
tesamorelin (brand name Egifta), a synthetic human growth
hormone-releasing factor developed by Theratechnologies,
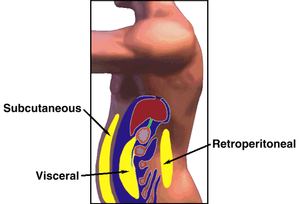
for the treatment of visceral abdominal fat accumulation
in people with HIV-related
lipodystrophy. The recommendation is based on Phase
3 study results showing that people taking tesamorelin
were nearly twice as likely to experience at least an
8% reduction in visceral fat. The main side effect of
concern is elevated blood glucose and diabetes. |
|
 |
 |
 |
 |
 |
 |
 |
By
Liz Highleyman
 Previous
research showed that recombinant human growth hormone (Serostim)
reduced visceral adipose or fat tissue, but it can lead to unacceptable
side effects including increased blood glucose, swelling, bone
pain, and carpal tunnel syndrome. In contrast, tesamorelin (formerly
TH9507) is a growth hormone-releasing factor that works by stimulating
the pituitary gland in the brain to secrete more growth hormone.
Previous
research showed that recombinant human growth hormone (Serostim)
reduced visceral adipose or fat tissue, but it can lead to unacceptable
side effects including increased blood glucose, swelling, bone
pain, and carpal tunnel syndrome. In contrast, tesamorelin (formerly
TH9507) is a growth hormone-releasing factor that works by stimulating
the pituitary gland in the brain to secrete more growth hormone.
Investigators hoped tesamorelin might provide benefits similar
to direct growth hormone administration but with fewer adverse
effects. As recently reported in the March
1, 2010 Journal of Acquired Immune Deficiency Syndromes,
patients receiving tesamorelin experienced an average visceral
fat reduction of 10.9% at 6 months, compared with just 0.6% among
placebo recipients; by 12 months, the reduction in the tesamorelin
arm reached 17.5%. These benefits were rapidly lost, however,
when patients stopped taking the drug.
Trunk fat, waist circumference, and waist-to-hip ratio all improved
significant in the tesamorelin arm and recipients reported improved
feelings about body image. Tesamorelin was associated with lower
total cholesterol and did not cause significant side effects including
blood glucose abnormalities, although levels of insulin-like growth
factor-1 (IGF-1) did increase significantly.
Christian Marsolais from Theratechnologies presented data at the
May 27 meeting showing that 57.4% of Phase 3 trial participants
who received tesamorelin experienced at least an 8% reduction
in visceral abdominal fat, compared with 29.3% of those taking
placebo. Clinical trial participants also testified about the
benefits of the drug.
Members of the committee discussed conflicting reports about tesmorelin's
effects on blood glucose. Theratechnologies researchers reported,
for example, that elevated blood glucose and diabetes were more
common among study participants during the first 6 months on tesamorelin,
though rates evened out by 12 months. An FDA safety review revealed
that while nearly half of tesamorelin recipients showed no blood
glucose elevations, about 17% had 3 or more elevated measurements,
with a higher risk among people with pre-existing glucose abnormalities.
Reviewers were also concerned that increased IGF-1 levels might
promote tumor development, but cancer rates were not higher among
tesamorelin recipients in Phase 3 trials. Although excess fat
is a risk factor for cardiovascular disease, differences in cardiovascular
event rates have also not been seen in tesamorelin trials to date.
Committee members determined that the benefits of tesamorelin
for HIV positive people with lipodystrophy outweigh the risks,
but they called for further studies to monitor its safety and
efficacy over a longer period.
The full FDA is not required to follow recommendations of its
advisory committees, but it usually does so. The agency has indicated
that it expects to complete its review and issue an opinion on
tesamorelin approval by July 27, 2010.
6/4/10
Sources
Theratechnologies. Theratechnologies announces positive vote by
FDA Advisory Committee for tesamorelin. Press release. May 27,
2010.
Food and Drug Administration. Tesamorelin
(Egrifta) Briefing Document. May 27, 2010.
L
Richwine. US panel backs Theratech drug for HIV patients. Reuters.
May 27, 2010.
