Medicare
Will Now Cover Facial Fillers for HIV Positive People Experiencing
Depression Due to Lipoatrophy
 |
 |
 |
 |
 |
 |
 |
| SUMMARY:
The Centers for Medicare & Medicaid Services announced
this week that Medicare will now pay for FDA-approved
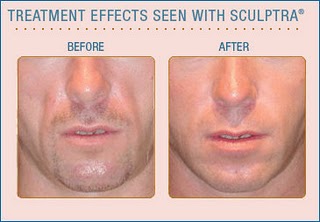
facial fillers such as poly-L-lactic acid (Sculptra)
and calcium hydroxylapatite (Radiesse) for people with
HIV who are experiencing symptoms of depression related
to facial lipoatrophy/lipodystrophy,
or fat loss, a side effect of certain older antiretroviral
drugs. |
|
 |
 |
 |
 |
 |
 |
 |
 Peripheral
lipoatrophy is characterized by wasting of subcutaneous fat in
the face and limbs. An adverse side effect associated with the
earliest nucleoside reverse transcriptase inhibitors including
zidovudine (AZT,
Retrovir), stavudine
(d4T, Zerit), and didanosine
(ddI, Videx), lipoatrophy can result in sunken cheeks that
reveal one's HIV status, causing feelings of stigma and psychological
distress. Medicare (which provides health coverage for people
age 65 and older) will now cover injectable facial fillers to
correct the condition. Peripheral
lipoatrophy is characterized by wasting of subcutaneous fat in
the face and limbs. An adverse side effect associated with the
earliest nucleoside reverse transcriptase inhibitors including
zidovudine (AZT,
Retrovir), stavudine
(d4T, Zerit), and didanosine
(ddI, Videx), lipoatrophy can result in sunken cheeks that
reveal one's HIV status, causing feelings of stigma and psychological
distress. Medicare (which provides health coverage for people
age 65 and older) will now cover injectable facial fillers to
correct the condition.
Below
is the recent announcement from the Centers for Medicare &
Medicaid Services describing the new policy.
|
Medicare
Expands Coverage for Treating Facial Lipodystrophy Syndrome
in People Living with HIV
March
23, 2010 -- The Centers for Medicare & Medicaid Services
(CMS) today announced its decision to cover facial injections
for Medicare beneficiaries who experience symptoms of depression
due to the stigmatizing appearance of severely hollowed
cheeks resulting from the drug treatment for Human Immunodeficiency
Virus (HIV). Today's decision is effective immediately.
Facial lipodystrophy (LDS) is a localized loss of fat from
the face, causing an excessively thin appearance in the
cheeks. In some cases, facial LDS may be a side effect of
certain kinds of medications (antiretroviral therapies)
that individuals receive as part of an HIV infection treatment
regimen.
The facial LDS can leave people living with HIV looking
gaunt and seriously ill, which may stigmatize them as part
of their HIV-infection status. Individuals who take these
medications and experience facial LDS side effects may suffer
psychological effects related to a negative self-image.
These effects may lead people living with HIV to discontinue
their antiretroviral therapies. The new decision allows
for treatment of individuals who experience symptoms of
depression due to the appearance changes from facial LDS.
The injections included in today's coverage decision are
"fillers" that have been approved by the U.S.
Food and Drug Administration (FDA) to be injected under
the skin in the face to help fill out its appearance specifically
for treatment of facial LDS. Data show that these injections
can improve patient self-image, relieve symptoms of depression,
and may lead to improved compliance with anti-HIV treatment.
"Today's decision marks an important milestone in Medicare's
coverage for HIV-infection therapies," said Barry M.
Straube, MD, CMS Chief Medical Officer and Director of the
Agency's Office of Clinical Standards and Quality. "Helping
people living with HIV improve their self-image and comply
with anti-HIV treatment can lead to better quality of life
and, ultimately, improve the quality of care that beneficiaries
receive."
|
The
final decision is posted on the CMS Web site.
3/26/10
Source
CMS Office of Public Affairs. Medicare Expands Coverage for Treating
Facial Lipodystrophy Syndrome in People Living with HIV. Media
release. March 23, 2010.
|
|
|
