Treatment
Interruption Spurs Fibrosis in HIV/HCV Coinfected People
SUMMARY
Stopping antiretroviral therapy led to liver fibrosis progression
in HIV/HCV coinfected people, an effect that was not fully
attributable to increased HIV viral load or reduced CD4
cell count. |
By
Liz Highleyman
Liver
damage due to chronic hepatitis C
virus (HCV) infection tends to progress more rapidly in
HIV/HCV coinfected
people compared to those with hepatitis C alone.
The
SMART
trial and other studies have shown that antiretroviral
therapy (ART) interruption can increase the risk of non-AIDS
conditions including heart, kidney, and liver disease, which
researchers have linked to increased immune activation and inflammation
related to resurgent HIV replication.
As described in the April
24, 2011, issue of AIDS, Julia Thorpe and fellow
investigators with the Canadian Coinfection Cohort Study (CTN222)
examined the effects of ART interruption on liver fibrosis progression
in HIV/HCV coinfected adults.
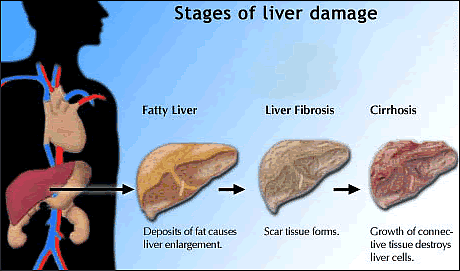
This
prospective analysis included 541 coinfected patients enrolled
between 2003 and 2009. ART interruption was defined as cessation
of all antiretroviral drugs for at least 14 days. Fibrosis was
assessed every 6 months, and participants were followed for
a median of just over 1 year.
The researchers estimated liver fibrosis using the APRI (aspartate
aminotransferase-to-platelet ratio index) method, a calculation
based on blood biomarkers. This method is considered less reliable
than the "gold standard" of liver biopsy, and possibly
also less so than the non-invasive transient elastometry (FibroScan)
technique. Participants had absent or minimal fibrosis at baseline.
The primary endpoint was an APRI score of at least 1.5, reflecting
significant fibrosis; a score of 2.0 or greater indicates fibrosis.
Results
 |
53
participants (10%) interrupted ART during follow-up (including
2 people who did so twice), with a median interruption duration
of 180 days. |
 |
The
same number (though not always the same 53 individuals) developed
significant fibrosis during a total 760 person-years of follow-up.
|
 |
After
accounting for potential confounding factors including age,
sex, CD4 cell count, HIV viral load, and baseline APRI score,
ART interruption had a hazard ratio of 2.52, or about a 2.5-fold
increased risk of fibrosis progression. |
 |
ART
interruption also more than doubled the risk of clinical liver
disease (hazard ratio 2.12), though this did not reach statistical
significance likely due to small numbers. |
Based
on these findings, the study authors concluded, "ART interruption
was associated with an increased risk of fibrosis progression in
HIV/HCV coinfection that was only partially accounted for by HIV
viral load and CD4 T-cell counts."
"Our findings suggest that liver disease progression observed
in ART-treated coinfected patients is partly due to the consequences
of treatment interruptions," they added.
"Studies to determine factors associated with antiretroviral
treatment interruption in coinfected patients would be beneficial
to assist clinicians in reducing treatment discontinuations, as
would studies aimed at understanding the underlying mechanisms driving
fibrosis in this setting," they elaborated in their discussion.
These findings support prior research indicating that early and
continuous antiretroviral treatment can slow or alleviate liver
damage related to chronic viral hepatitis in people with HIV.
Investigator affiliations: Department of Medicine, Divisions
of Infectious Diseases/Immunodeficiency, Royal Victoria Hospital,
Canada; Department of Epidemiology & Biostatistics, McGill University,
Montreal, Quebec, Canada.
4/26/11
Reference
Thorpe, Juliaa; S Saeed, E Moodie, and MB Klein (for the Canadian
Co-infection Cohort Study; CTN222). Antiretroviral treatment interruption
leads to progression of liver fibrosis in HIV-hepatitis C virus
co-infection. AIDS 25(7):967-975 (abstract).
April 24, 2011.
|
