|
 Lexiva
- Important Safety Information Lexiva
- Important Safety Information
| |
You
should not take LEXIVA if you have had
an allergic reaction to LEXIVA or AGENERASE®
(amprenavir). |
| |
High
blood sugar, diabetes or worsening of
diabetes, and bleeding in hemophiliacs
have occurred in some patients taking
protease inhibitors. |
| |
When
you start taking HIV medicines, your
immune system may get stronger and could
begin to fight infections that have
been hidden in your body, such as pneumonia,
herpes virus, or tuberculosis. If you
have new symptoms after starting your
HIV medicines, be sure to tell your
doctor. |
| |
Changes
in body fat may occur in some patients
taking antiretroviral therapy. The cause
and long-term health effects of these
conditions are not known at this time.
|
| |
Skin
rashes can occur in patients taking
LEXIVA. Rarely, rashes were severe or
life threatening. |
| |
Opportunistic
infections can develop when you have
HIV and your immune system is weak.
It is very important that you see your
healthcare provider regularly while
you are taking LEXIVA to discuss any
side effects or concerns. |
| |
Most
common side effects in clinical studies
were diarrhea, headache, nausea, rash,
and vomiting. In most cases, these side
effects did not cause people to stop
taking their medicine. |
 Lexiva
- Drug Interactions Lexiva
- Drug Interactions
| |
LEXIVA
should not be taken with: AGENERASE®
(amprenavir), Halcion® (triazolam),
ergot medications (Cafergot®,
Migranal®, D.H.E. 45®,
and others), Propulsid® (cisapride),
Versed® (midazolam), Orap®
(pimozide), Zocor® (simvastatin),
Mevacor®, (lovastatin), Rifadin®
(rifampin), Rescriptor® (delavirdine
mesylate), or St. John's wort (Hypericum
perforatum). If you are taking Norvir®
(ritonavir), you should not take Tambocor®
(flecainide), or Rythmol®
(propafenone hydrochloride). |
| |
Serious
and/or life-threatening events could
occur between LEXIVA and other medications,
including Cordarone® (amiodarone),
lidocaine (intravenous only), Elavil®
(amitriptyline HCl) and Tofranil®
(imipramine pamoate), tricyclic antidepressants,
and Quinaglute® (quinidine). |
| |
Women
who use birth control pills should choose
a different kind of contraception. LEXIVA
can affect the safety and effectiveness
of birth control pills. |
| |
Patients
taking Viagra® (sildenafil
citrate) or LEVITRA® (vardenafil
HCl) with LEXIVA may be at an increased
risk of side effects. |
| |
This
list of drug interactions is not complete.
Be sure to tell your healthcare provider
about all medicines you are taking or
plan to take, including over-the-counter
drugs, vitamins, and herbals. |
 Lexiva
- Dosing Lexiva
- Dosing
Your healthcare provider may prescribe LEXIVA
for you either once a day or twice
a day, based on your HIV treatment history.
Often LEXIVA can be taken at the same time
you take your other HIV medicines.
When LEXIVA is prescribed with ritonavir,
this is known as "boosting" the dose of
LEXIVA. Boosting with ritonavir increases
the amount of LEXIVA in your body. And boosting
may give your healthcare provider the option
of prescribing LEXIVA once or twice a day.
LEXIVA, with or without ritonavir, is only
4 pills a day.
Whatever regimen you are prescribed, remember:
always take your HIV medicines exactly as
your healthcare provider tells you to.
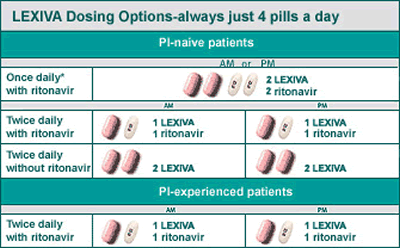
*Once daily with ritonavir
is not recommended for PI-experienced patients.
 Lexiva
- Resistance Profile Lexiva
- Resistance Profile
ART-naive
patients
secondary
protease mutations

APV30002
(SOLO) a randomized, open-label study of
LEXIVA/r (1400/200 mg) QD vs NFV (1250 mg)
BID in combination with ABC/3TC BID in 649
ART-naive patients.
Because the potential for HIV cross-resistance
among protease inhibitors has not been fully
explored, it is unknown what effect therapy
with LEXIVA will have on the activity of
subsequently administered protease inhibitors.
Clinical relevance of resistance data is
currently being evaluated.
*
Excludes patients without paired genotypes
at baseline and at the time that virologic
failure was first detected.
Common natural polymorphisms
in the absence of other PRO mutations are
excluded: NFV n = 3 [M36 m/l, K20k/m, L10l],
LEXIVA/r n = 1 [V77v/i].
ART-naive
patients
LEXIVA,
BID limited cross-resistance
APV30001
(NEAT) a randomized, open-label study of
LEXIVA (1400 mg) BID vs NFV (1250 mg) BID
in combination with ABC/3TC BID in 249 ART-naive
patients.
Excludes patients without paired genotypes
at baseline and at the time that virologic
failure was first detected.
§ Common natural polymorphisms
in the absence of other PRO mutations are
excluded: LEXIVA n = 1 [A71a/t].
|