Early
Analysis Finds Good Outcomes with Once-daily Dosing of HCV Protease Inhibitor
TMC435
 |
 |
 |
 |
 |
|
| SUMMARY:
Tibotec's investigational hepatitis C virus (HCV) NS3/4A protease
inhibitor, TMC435, produced good viral suppression in previously
untreated genotype 1 patients when taken once-daily in combination
with pegylated interferon plus ribavirin for 12 or 24 weeks,
according to interim results from the PILLAR study presented
this week at the American Association for the Study of Liver
Diseases "Liver Meeting" (AASLD
2010) in Boston. |
|
|
 |
 |
 |
 |
By
Liz Highleyman
Current
standard therapy for genotype 1 chronic hepatitis C consists of pegylated
interferon (Pegasys or PegIntron) plus ribavirin for 48 weeks. Interferon-based
therapy can cause difficult side effects, however, and only about half
of people with this hard-to-treat HCV genotype achieve a cure with the
first course of treatment.
Novel oral drugs that directly target steps of the viral lifecycle --
such as HCV protease and polymerase inhibitors -- are currently in development.
Most studies to date have looked at these agents in combination with
pegylated interferon plus ribavirin, though some have started to look
at all-oral combinations.
After the Phase 2a OPERA trial demonstrated promising results (also
reported at AASLD 2010), the Phase 2b PILLAR study (TMC435-C205 or NCT00882908)
was designed to assess the safety and efficacy of TMC435
in combination with pegylated interferon alfa-2a (Pegasys) plus ribavirin
in treatment-naive patients with HCV genotype 1.
This multinational trial included a total of 386 participants. Just
over half were men, most (94%) were white, and the median age was about
47 years. About 45% had HCV genotype 1a, while 55% had genotype 1b.
More than 85% had high HCV RNA viral load at baseline (>800,000
IU/mL) and 14% had advanced liver fibrosis (stage F3); those with cirrhosis
(stage F4) were not eligible. About 30% had the favorable C/C IL28B
gene pattern, a recently discovered marker that predicts good response
to interferon.
Table 4. Mean (± SE) baseline and Day 7 hepatic parameters
for each GT cohort.
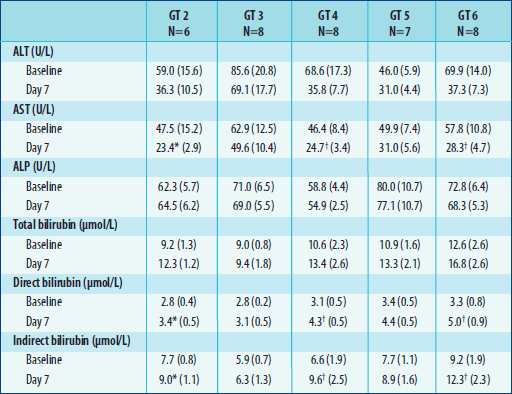
Participants
were randomly allocated into 5 treatment arms:
 |
75
mg once-daily TMC435 + 180 mcg/week pegylated interferon + 1000-1200
mg/day weight-adjusted ribavirin for 12 weeks, followed by pegylated
interferon + ribavirin alone for another 12 weeks (TMC12-PR24 75mg); |
 |
150
mg once-daily TMC435 + same doses of pegylated interferon + ribavirin
for 12 weeks, followed by pegylated interferon/ribavirin for another
12 weeks (TMC12-PR24 150mg); |
 |
75
mg once-daily TMC435 + same doses of pegylated interferon + ribavirin,
all taken for 24 weeks (TMC24-PR24 75mg); |
 |
150
mg once-daily TMC435 + same doses of pegylated interferon + ribavirin,
all taken for 24 weeks (TMC24-PR24 150mg); |
 |
Standard
therapy consisting of the same doses of pegylated interferon + ribavirin
for 48 weeks, with placebo (comparable to TMC435) for the first
24 weeks (Placebo24-PR48). |
Patients
in the TMC435 arms who achieved HCV RNA < 25 IU/mL at week 4, as
well as undetectable at weeks 12, 16, and 20, were allowed to end all
treatment at week 24. All other participants continued pegylated interferon
+ ribavirin through 48 weeks.
The investigators measured HCV levels regularly throughout treatment
and follow-up. The primary endpoint will be sustained virological response
at 24 weeks after completion of treatment, or SVR24, which is considered
a cure. An interim measure -- sustained response at 12 weeks after finishing
treatment, or SVR12 -- was reported at the AASLD meeting. The researchers
also assessed viral breakthrough, safety, and tolerability.
Results
 |
Rapid
virological response (RVR), or undetectable HCV RNA < 25 IU/mL
at week 4 of therapy, was significantly more likely in all TMC435
arms compared with standard therapy: |
| |
 |
TMC12-PR24
75mg: 77%; |
 |
TMC12-PR24
150mg: 76%; |
 |
TMC24-PR24
75mg: 68%; |
 |
TMC24-PR24
150mg: 79%; |
 |
Placebo24-PR48:
5%. |
|
 |
Rates
of complete early virological response (EVR), or undetectable HCV
RNA < 25 IU/mL at week 12, were high in all TMC435 arms: |
| |
 |
TMC12-PR24
75mg: 91%; |
 |
TMC12-PR24
150mg: 94%; |
 |
TMC24-PR24
75mg: 96%; |
 |
TMC24-PR24
150mg: 97%; |
 |
Placebo24-PR48:
58%. |
|
 |
Response
rates at week 24 (end-of-treatment for the TMC435 arms) remained
high in the TMC435 arms, with standard therapy showing a gain: |
| |
 |
TMC12-PR24
75mg: 94%; |
 |
TMC12-PR24
150mg: 94%; |
 |
TMC24-PR24
75mg: 97%; |
 |
TMC24-PR24
150mg: 95%; |
 |
Placebo24-PR48:
82%. |
|
 |
12-week
sustained response, or SVR12, was reported for about 40% of patients
who had reached this point in post-treatment follow-up (not yet
available for standard therapy patients, who will complete treatment
at week 48): |
| |
 |
TMC12-PR24
75mg: 97%; |
 |
TMC12-PR24
150mg: 89%; |
 |
TMC24-PR24 75mg: 93%; |
 |
TMC24-PR24
150mg: 88%; |
|
 |
79%
to 86% of patients in TMC435 arms were able to end treatment at
week 24, according to protocol-defined response criteria. |
 |
The
combined rate of viral breakthrough in the TMC435 arms was 4.9%
vs 3.5% in the standard therapy arm; breakthrough rates were higher
in the arms that received TMC435 for 12 rather than 24 weeks (6.4%-7.8%
vs 2.5%-2.7%, respectively). |
 |
Adding
TMC435 to pegylated interferon/ribavirin increased response rates
for patients with all IL28B genotypes. |
 |
ALT
and AST liver enzyme levels decreased significantly in all treatment
arms. |
 |
The
most common adverse events were headache (46% in TMC435 arm vs 51%
in placebo arm) and fatigue (42% vs 47%, respectively). |
 |
Overall
frequency of adverse events did not differ significantly between
TMC435 and placebo arms. |
 |
7.1%
of patients in the TMC435 arms and 7.8% in the placebo arm experienced
adverse events leading to treatment discontinuation, not a significant
difference. |
 |
There
were no significant differences in frequencies of gastrointestinal
symptoms, rash (28.5% vs 27.3%, respectively), or anemia (19.4%
vs 20.8%, respectively). |
 |
Patients
in the 150 mg TMC435 dose arms, however, were significantly more
likely to experience mild and reversible elevations in bilirubin. |
"This
interim analysis demonstrates that TMC435 in addition to pegylated interferon/ribavirin
achieves RVR and complete EVR for the majority of patients," the
researchers concluded. "Available data indicate that high SVR12
rates are observed in patients who completed therapy."
"In this analysis TMC435 was well tolerated in all treatment arms,"
they added.
"Chronic
infection with HCV is a leading cause of cirrhosis, liver cancer, and
liver transplantation worldwide," lead investigator Michael Fried
from the University of North Carolina at Chapel Hill said in a press
release issued by Tibotec. "We are extremely encouraged by these
data for TMC435."
Researchers
also reported results from a Phase 2a proof-of-concept study evaluating
200 mg once-daily TMC435 monotherapy for 7 days in treatment-naive people
with HCV genotypes 2 through 6, which (with the possible exception of
4) respond better to interferon than genotype 1.
TMC435 monotherapy
for 7 days "showed potent antiviral activity, with highest antiviral
activity against genotype 4 and genotype 6, followed by genotype 5 and
genotype 2," they concluded. However, "[n]o antiviral activity
was seen against genotype 3." All adverse events were mild-to-moderate,
with no discontinuations during the 7-day period.
TMC435 is
also being evaluated in the international Phase 2b ASPIRE study for
treatment-experienced HCV genotype 1 patients who did not achieve sustained
response with a previous course of pegylated interferon/ribavirin.
Investigator
affiliations:
Fried study (PILLAR): Director of Hepatology, University of North Carolina
at Chapel Hill, Chapel Hill, NC; Hospital Vall d'Hebron and Ciberehd,
Barcelona, Spain; St. Vincent's Hospital, Sydney, NSW, Australia; Allgemeines
Krankenhaus der Stadt Wien, Wien, Austria; Weill Cornell Medical College,
New York, NY; Hopital Beaujon, Clichy, Paris, France; Klinikum der Johann-Wolfgang-Goethe-Universitat--Med.
Klinik I, Frankfurt, Germany; Tibotec BVBA, Beerse, Belgium; Tibotec
Inc., Titusville, NJ.
Lenz study (OPERA-1): Tibotec BVBA, Beerse, Belgium; Tibotec Inc, Yardley,
PA.
Moreno study: Department of Gastroenterology and Hepatopancreatology,
Erasme Hospital, Brussels, Germany; Medizinische Klinik m. S. Hepatologie
und Gastroenterologie Charité, Campus Virchow-Klinikum, Universitatsmedizin
Berlin, Berlin, Germany; Department of Medicine, Siriraj Hospital, Mahidol
University, Bangkok, Thailand; Chiang Mai University, Chiang Mai, Thailand;
Department of Gastroenterology and Hepatology, Ghent University Hospital,
Ghent, Belgium; Department of Medicine I, J.W. Goethe University Hospital,
Frankfurt, Germany; Tibotec BVBA, Mechelen, Belgium; Tibotec Inc., Titusville,
NJ.
11/5/10
References
MW Fried,
M Buti, GJ Dore, and others. Efficacy and safety of TMC435 in combination
with peginterferon alfa-2a and ribavirin in treatment-naive genotype-1
HCV patients: 24-week interim results from the PILLAR study. 61st Annual
Meeting of the American Association for the Study of Liver Diseases
(AASLD 2010). Boston, October 29-November 2, 2010. Abstract
LB-5.
O Lenz,
L Vijgen, T Lin, and others. Virologic analysis of genotype-1-infected
patients treated with once-daily TMC435 during the Optimal Protease
inhibitor Enhancement of Response to therApy (OPERA)-1 study. 61st Annual
Meeting of the American Association for the Study of Liver Diseases
(AASLD 2010). Boston, October 29-November 2, 2010. Abstract
812.
C Moreno,
T Berg, T Tanwandee, and others. A Phase IIa, open-label study to assess
the antiviral activity of TMC435 monotherapy in patients infected with
HCV genotypes 2-6. 61st Annual Meeting of the American Association for
the Study of Liver Diseases (AASLD 2010). Boston, October 29-November
2, 2010. Abstract
895.
Other
Source
Tibotec.
Week 24 interim results from phase 2b PILLAR study to be presented as
late-breaker at AASLD. Press release. October 30, 2010.