Studies Look at Long-term Efficacy Safety of Tenofovir (Viread/Truvada/Atripla), including Kidney Toxicity and Bone Loss By
Liz Highleyman Several presentations last month at the 48th International Conference on Antimicrobial Agents and Chemotherapy (ICAAC 2008) provided new data on tenofovir's long-term efficacy and safety, in particular the occurrence of kidney and bone problems, which have been attributed to tenofovir in some previous studies. Atripla Efficacy and Side Effects In study AI26607, participants on stable antiretroviral therapy with HIV suppression < 200 cells/mm3 for at least 3 months were randomly assigned to stay on their current HAART regimen (n = 97) or switch to Atripla (n = 203), which provides a full NNRTI-based antiretroviral regimen in a single once-daily pill. Most patients (88%) were men, about two-thirds were white, and the mean age was 43 years. The median baseline CD4 count was about 540 cells/mm3 and 96% had HIV RNA < 50 copies/mL. Results
In conclusion, the investigators wrote, "High and comparable rates of virologic suppression were maintained with [efavirenz/emtricitabine/tenofovir] vs [baseline regimen], regardless of type of prior antiretroviral therapy." "Mild, transient nervous system symptoms occurred with [efavirenz/emtricitabine/tenofovir], particularly with prior PI-based antiretroviral therapy," they added. Orlando Immunology Center, Orlando, FL; Denver Infectious Disease Consultants, Denver, CO; Gilead Sciences, Inc., Foster City, CA; Bristol-Myers Squibb, Princeton, NJ. Long-term Atripla
In Gilead's Study 934, participants starting antiretroviral therapy for the first time were randomly assigned to receive Truvada or the Combivir (zidovudine/lamivudine) coformulation pill, both in combination with efavirenz (Sustiva). After 144 weeks, 286 patients from both arms switched to the Atripla pill and continued follow-up. Most participants in the extension study (88%) were men, 65% were white, the mean age was 40 years, the median CD4 count was 535 cells/mm3, and about 95% had HIV RNA < 50 copies/mL. Results
"Switching Truvada or Combivir + efavirenz to a single tablet once-daily regimen of [efavirenz/emtricitabine/tenofovir] was well-tolerated and resulted in maintenance of virologic suppression through 48 weeks," the researchers concluded. However, they added, "In patients on Combivir + efavirenz for 3 years, switching to Atripla did not significantly improve limb fat after 48 weeks." Orlando
Immunology Ctr., Orlando, FL; Chelsea & Westminster Hosp, London, UK; Johns
Hopkins Univ, Baltimore, MD; Univ Hosp La Paz, Madrid, Spain; Gilead Sciences,
Foster City, CA.
Another
research team looked at changes in bone mineral density among patients taking
tenofovir/emtricitabine.
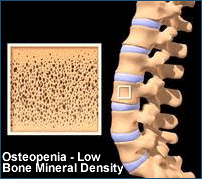
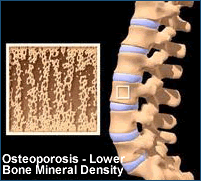
Studies have shown that people with HIV have an elevated risk of bone loss (osteopenia
and the more serious osteoporosis), but the reasons for this are not
fully understood. The
investigators hypothesized that tenofovir might contribute to abnormal calcium
metabolism via secondary hyperparathyroidism, or excess production of parathyroid
hormone, which regulates calcium and phosphate levels. In
this prospective cross-sectional study, they reviewed medical records and interviewed
51 HIV positive men on HAART with normal kidney function (GFR > 60) and normal
serum calcium levels; 34 were taking antiretroviral regimens containing tenofovir
plus emtricitabine (Emtriva;
also in the Truvada and Atripla
pills).
Based
on these findings, the investigators concluded, "[Tenofovir/emtricitabine]
appears to be associated with [secondary hyperparathyroidism] in patients with
low vitamin D, but not in patients with sufficient vitamin D, suggesting that
adequate doses of vitamin D supplements along with tenofovir may prevent [secondary
hyperparathyroidism], a serious condition linked to bone loss and cardiovascular
disease." They
recommended that patients taking tenofovir should have their vitamin D and parathyroid
hormone levels checked, and urged further research to investigate whether prophylactic
use of vitamin D and calcium supplements might prevent increases in parathyroid
hormone and preserve bone in this population.
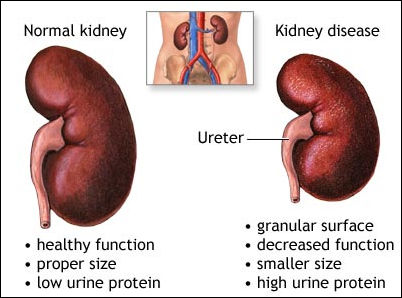
Researchers
at Johns Hopkins assessed whether tenofovir was associated with renal dysfunction
in patients starting HAART for the first time. They compared all treatment-naive
patients with an estimated GFR > 50 ml/min/1.73m2 (by MDRD) who started either
tenofovir (n=201) or alternative nucleoside reverse transcriptase inhibitors (NRTIs)
(n=231) after January 2002, analyzing the time to 25% and 50% decline in GFR.
In conclusion, the researchers stated, "Our results emphasize the importance of having a control group who receive an alternative NRTI, since a modest decline in [GFR] was observed among patients taking both tenofovir and [alternative NRTIs]." "Our results support use of tenofovir within the initial antiretroviral therapy regimen," they concluded. "Our data also suggest that GFR should be monitored more closely in older patients, those with CD4 counts < 200 cells/mm3, when hypertension is present, and when a [ritonavir-boosted PI] is used.'" Johns Hopkins Univ. School of Medicine, Baltimore, MD. In a related report, researchers in Philadelphia also compared kidney function in patients taking tenofovir with NNRTIs (n = 110) versus boosted PIs (n = 140). At baseline, study participants had been on HAART for at least 3 months. Here too, a majority were men and about two-thirds were African-American. Overall, 3 patients changed their regimens due to renal problems. However, the investigators observed no evidence of significant changes in GFR from baseline during the first 2 years of therapy (P > 0.05). Multivariate analysis including age, sex, hypertension, diabetes, and treatment-naive or experienced status did not alter these findings. "There was no change in creatinine clearance with either a boosted PI or NNRTI," they concluded. "Sub-analysis of the [African-American] population did show a significant difference in first year of therapy; however, these differences were not statistically different over the second year of treatment." Jefferson
Med. College, Philadelphia, PA; Drexel Univ. College of Med., Philadelphia, PA. E. DeJesus, A Pozniak, J Gallant, and others. The 48-Week Efficacy and Safety of Switching to Fixed-Dose Efavirenz/Emtricitabine/Tenofovir DF in HIV-1-Infected Patients Receiving HAART. 48th International Conference on Antimicrobial Agents and Chemotherapy (ICAAC 2008). Washington, DC. October 25-28, 2008. Abstract H-1235. K Childs, S Fishman, K Bateman, and others. Should Vitamin D Be Prescribed with Tenofovir/FTC? 48th International Conference on Antimicrobial Agents and Chemotherapy (ICAAC 2008). Washington, DC. October 25-28, 2008. Abstract H-2300. R Moore and J Gallant. Renal Function after Use of Tenofovir as Part of the Initial ART Regimen. 48th International Conference on Antimicrobial Agents and Chemotherapy (ICAAC 2008). Washington, DC. October 25-28, 2008. Abstract H-2297. WR Short, P Solari, and B Kaplan. Comparison of Renal Function on NNRTI vs. Boosted PI based Tenofovir based Regimens. 48th International Conference on Antimicrobial Agents and Chemotherapy (ICAAC 2008). Washington, DC. October 25-28, 2008. Abstract H-2298. |
The
material posted on HIV and Hepatitis.com about ICAAC 2008 and IDSA 2008 is not
approved by nor is it a part of ICAAC 2008 or IDSA 2008. |
![]()